REMPI is an acronym for Resonant Enhanced MultiPhoton Ionization.
REMPI works by utilizing a high photon density to excite molecules into an excited state followed by ionization. This is a stepwise ionization that requires a minimum of 2 photons. The Ionization rate is higher when the photons are in resonance with an intermediate state. Ions are selected and detected using a mass spectrometer, most often a Time-Of-Flight (TOF) is used.
To record a REMPI spectrum a tunable light source, usually a dye laser, is needed and the ion yield is plotted (Y-axis) versus wavelength (X-axis).
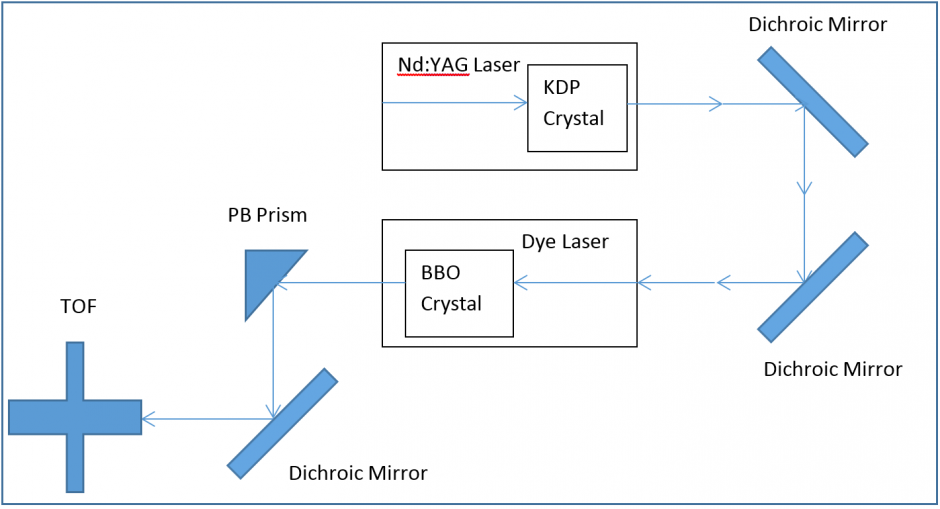
Figure 1. Block Diagram highlighting key optical features of our REMPI setup. 1064 nm light is produced using a pulsed Nd:YAG laser, which is then frequency doubled to 532nm to pump a dye laser. The dye laser selectively emits at a wavelength dependent on the dye (560 ±5 nm with Pyrromethane 597), and then the output of the dye is frequency doubled. (≈280nm). Tunable UV light is then used to ionize the sample.
Since absorption of a photon is required to have a good ion yield, a REMPI spectrum is expected to show the same feature as an absorption spectrum. REMPI is basically a special case of excitation spectrum. It is important to note that selection rules are different for a multiple photon process. This is actually an advantage of REMPI, transitions that are forbidden using a single photon are not necessarily forbidden when more than one photon is used.
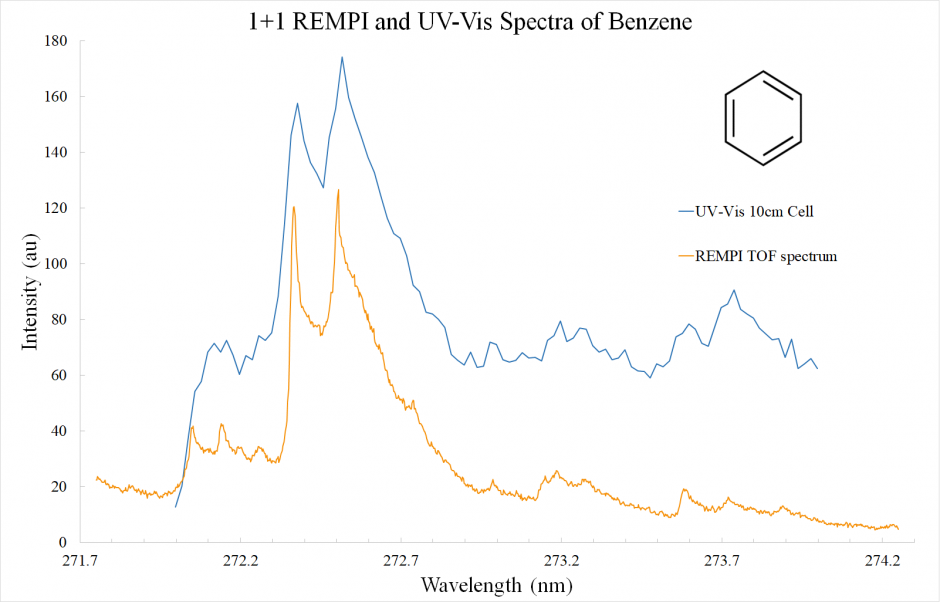
Figure 2. UV VIS (blue) and REMPI Spectrum (orange) of Benzene (C6H6). The two spectra show the same features.
Another advantage of REMPI, is that multiple photons can be absorbed, allowing for ionization at less energetic wavelengths than expected. Both styrene and N2 spectra have been collected using REMPI at around the same wavelength (280 nm). However, the ionization energy is approximately 8 eV for styrene, and for 15.6 eV for Nitrogen. At this wavelength styrene undergoes a 1+1 transition and N2 undergoes a 2+2 transition. Twice as many photons are used to ionize N2
In the diagrams below, each arrow represents the energy of a single photon, and the lines represent different atomic or molecular electronic states. Note how the arrows are equal in length, all photons emitted by the laser have the same energy.
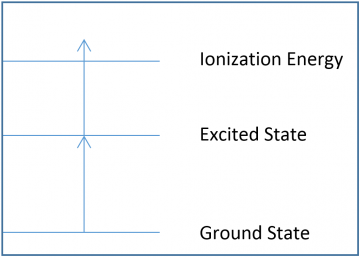
Figure 3: A 1+1 photoionization diagram. 1+1 photoionization require one photon to reach the excited electronic state, and another photon to ionize the sample.
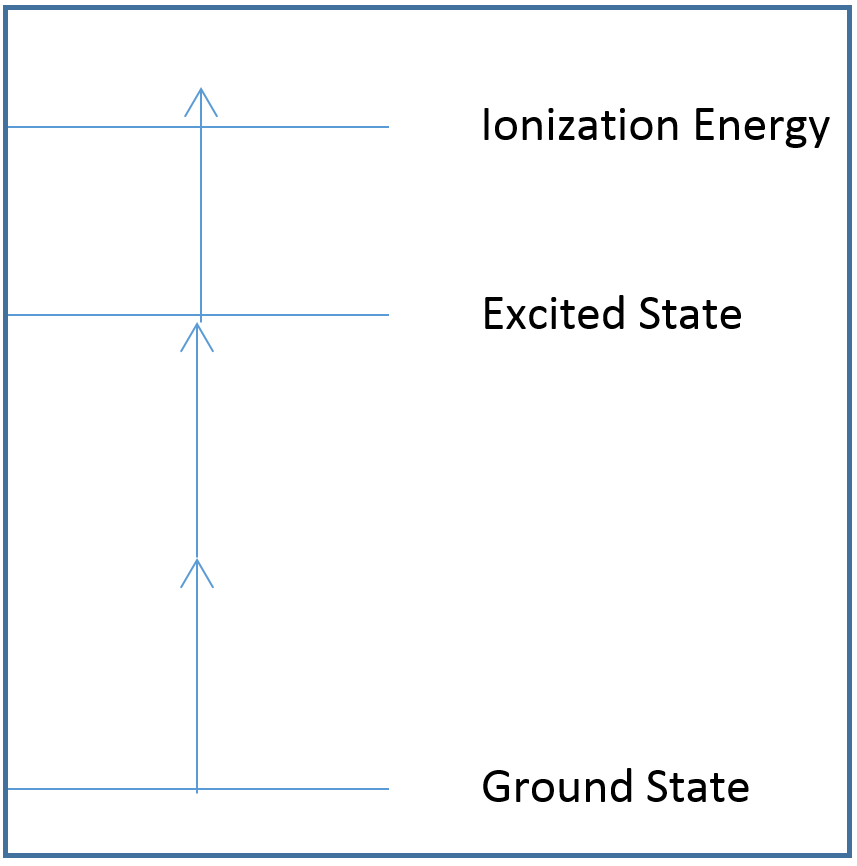
Figure 4: A 2+1 photoionization diagram. 2+1 photoionization requires two photons to reach the excited electronic state, and another photon to ionize the sample.
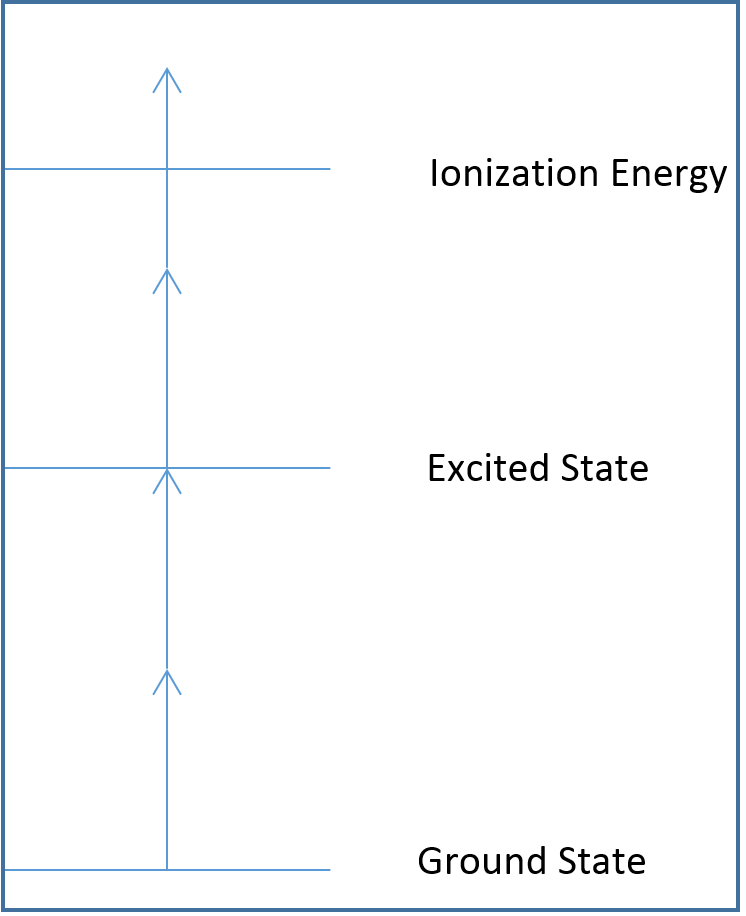
Figure 5: A 2+2 photoionization diagram. 2+2 requires two photons to reach the excited electronic state, and two more photons to ionize the sample.